Print Files: A4 Size (pdf), Text (txt).
Conventional treatment of tooth decay involves removal of the affected part of the tooth followed by filling of the hole with a material such as resin or metal alloy [1]-[4]. This method is less than optimal for microscopic early decay [5],[6] because some of the healthy tooth must also be removed to enable fixing of the filling. Here we describe a new dental paste that achieves rapid repair of early tooth decay.
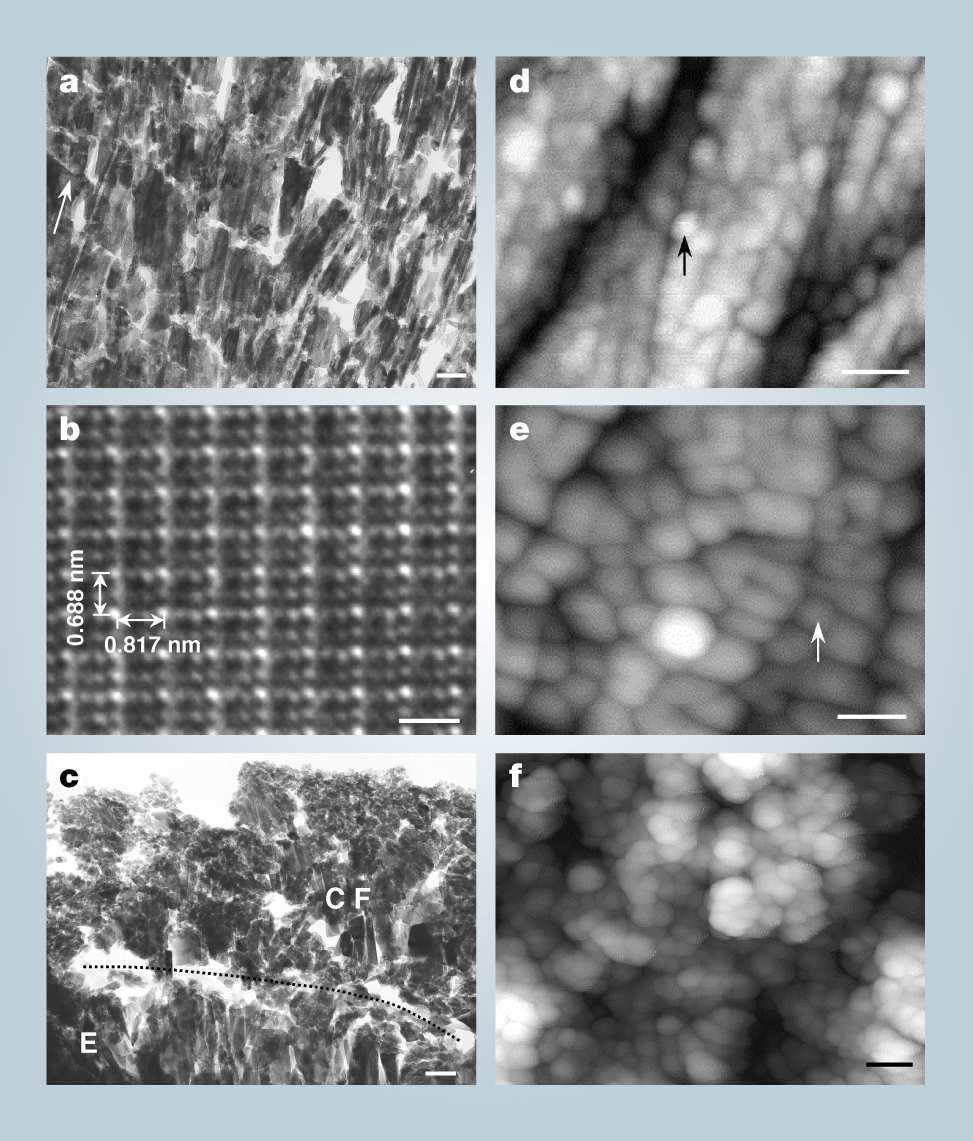
The outer layer of a human tooth is called the enamel. It is 1-1.5 mm thick and is composed of hydroxyapatite (HAP) crystals. Early tooth decay involves microscopic damage to the enamel (holes < 50 μm deep) by acid-forming bacteria, which cannot be repaired by simple filling materials because perfect adhesion with the enamel does not occur due to differences of chemical composition and structure. Our paste grows HAP crystals, which are exactly like those in natural enamel, at the affected site within 15 min (for the method and examples of treatment, see supplementary information). Figure a shows a transmission electron microscopy (TEM) image of a lower premolar repaired with our paste. The interface between the repaired layer and the enamel shows no clear gap. The repaired layer contains elongated crystals (100-400 nm long and 20-80 nm wide) that are regularly orientated to the tooth surface and have grown across the interface, showing that the paste has strongly bonded to the tooth enamel. Atomic resolution TEM of a crystal (Fig. b) shows a pattern that is consistent with the known lattice pattern of HAP: 0.688 nm for the short axis (c) of the crystal (arrow), and 0.817 nm for the long axis (a). Then X-ray photoelectron spectroscopy confirms that these are fluoridated HAP crystals growing parallel to the tooth surface [7]. The repaired enamel layer shows a high durability and acid tolerance (see supplementary information). For comparison with treatment using acidic phosphate fluoride (APF) solution, an alternative for the repair of early decay, look at Fig. c. The TEM image shows an irregular layer [8],[9] of calcium fluoride less than 1 μm thick covering the enamel, with a clear gap at the interface (arrow and dotted line).
Time-lapse atomic force microscopy (AFM) showed that the apatite crystals of the original tooth enamel (Fig. d) are slightly dissolved by application of the paste, but quickly grow again using the paste as a source of minerals. This dissolution and re-growth occur due to the strong acidity (pH < 2) of the paste, and the result is a continuous structure of new crystals extending from the original enamel to the repaired layer. The new fluoridated HAP crystals cover the whole tooth surface in a densely packed array after 3 min (Fig. e), and are stacked three-dimensionally after 15 min (Fig. f). The acidic paste contributes to rapid growth of the crystals by breaking down calcium phosphate clusters, the growth unit of HAP, to liberate calcium and phosphate ions as reported previously [10],[11].
We demonstrated that the paste can repair and prevent early decay by constructing "synthetic enamel". When used on patients, the paste should not be allowed to contact the gums to prevent inflammation due to its acidity and high concentration of hydrogen peroxide, although similar strengths are already used in dental clinics.
The mother solution was prepared by mixing a 35% H2O2 aqueous solution with an 85% solution of H3PO4 at a volume ratio of 4:1. The paste was made by adding 2 ml of mother solution to 1.5 g of fluorized-apatite powder, which was prepared by mixing 1 g of Ca-deficient HAP powder (Ube Materials Co., Japan. Ca/P=1.64) with 100 ml of 200 mM NaF solution. The NaF solution with dissolved HAP was stirred for 1 h at 60oC, and the precipitate (F-HAP) was filtered, washed with pure water and dried at 110oC for 24 h. In the treatment, a tiny amount of mother solution was brushed on the affected part, and the paste was quickly applied before the solution dried without any mechanical removal. Within 15 min of treatment, approximately 20 μm thick of F-HAP layer is constructed on the affected part. APF treatment was done with APF solution (480 mM NaF, pH=3.5), which is commonly used in dental clinics, following the treatment protocol. The durability was tested using a brushing machine and a commercially available toothbrush and toothpaste at a speed of 150 rev./min, a load force of 200 g, brushing amplitude of 30 mm, and 10000 times of brushing. The acid tolerance was tested using acidic simulated saliva conformed to the British Standard Specification for Safety Hardnesses; it contained 77 mM NaCl, 4.0 mM KCl, 2.1 mM Na2SO4, 7.5 mM NH4Cl, 3.3 mM urea, and 33 mM lactic acid with a pH of 4.5 adjusted by NaOH at 25oC. The dissolution rates of the re-grown layer and enamel were compared using laser scanning confocal microscopy.

Legend of Figure a, b, c, d, e, f
a TEM image around interface between re-grown layer and enamel. Pillar crystals grew continuously, and no discontinuous boundary was observed. Upper part of figure corresponds to re-grown layer, and lower, enamel region. Arrow indicates the direction of tooth surface. Scale bar is 100 nm.
b Atomic Image of grown crystal. Scale bar is 1 nm.
c TEM Image of APF treated tooth. CaF2 particles cover the enamel apatite crystals; a clear structural gap is seen between them. Scale bar is 100 nm.
d AFM image of original tooth enamel. Polygonal blocks (arrow) seen on the surface are apatite single crystals. Scale bar is 50 nm.
e Image of newly grown F-HAP crystals (arrow) after 3 min of paste repair. Scale bar is 50 nm.
f Surface after repair completed (15 min). Grown F-HAP crystals are stacked three-dimensionally. Scale bar is 100 nm.